AstraZeneca Osimertinib as a first-line therapy for NSCLC has been approved in China, which will further benefit domestic patients.
Release time:
2019-09-19 11:22
2019Year8Month31On the 1st, the State Drug Administration (NMPA) official website shows that osimertinib developed by AstraZeneca is used for epidermal growth factor receptor (EGFR) Locally advanced or metastatic non-small cell lung cancer with positive gene mutation (NSCLC) listing application for first-line treatment of patients (JXHS1800054/5) The approval is completed and the certification stage is entered, marking the official role of Osimertinib.NSCLCThe first-line therapy is approved in China.
Osimontinib Mesylate Tablets (Tagrisso®,AZD9291) is AstraZeneca's No.3Generation of oral, irreversible selectiveEGFRMutant inhibitors, the first to be listed in the world and the first to be approved in ChinaEGFR T790MTargeted oncology drugs for mutation-positive locally advanced or metastatic non-small cell lung cancer.
In China, lung cancer is a malignant tumor with high morbidity and mortality, and the number of new lung cancer patients per year exceeds73Ten thousand people. The number of non-small cell lung cancer patients in China is about30-40%OccurrenceEGFRmutation, while receivingEGFR-TKIDrugsEGFRMutation patients have about 2/3T790MMutation and drug resistance, leading to disease progression again, patients need to get new treatment. Compared to the first and second generationEGFR-TKIThe third generation.EGFROsimertinib has two significant advantages:1.To lead the first generationEGFR-TKIDrug failureT790MThe mutation remains valid;2.Third GenerationTAKESThe specificity is improved, resulting in effective and precise suppression and reduced side effects.
In recent years, based on rigorous evidence-based medical evidence, osimertinib from17Year3The month of listing in the country became the only approved.EGFR-T790Mmutant non-small cell lung cancer treatment drugs,18Year10entered our health insurance in January, and is now amongEGFRThe first-line drug for mutant metastatic non-small cell lung cancer has gradually become aEGFRGene positive mutation is the main weapon of lung cancer treatment.
Globally, several authoritative clinical practice guidelines strongly recommend osimertinib as first-line therapyEGFRlate positiveNSCLCThe drug of choice.2018The U.S. Food and Drug Administration (FDA) basedFLAURAStudy Results, Osimertinib Approved for First-Line TherapyEGFRpositive non-small cell lung cancer patients.2019At the beginning of the year, the National Comprehensive Cancer Network (NCCN) guidelines further recommend osimertinib as aEGFRPositive non-small cell lung cancer patients with first-line medication as the preferred recommend, and in gefitinib, erlotinib and so on.2018Year10Month European Society of Oncology (ESM) clinical practice guidelines also incorporate osimertinibEGFRPositive non-small cell lung cancer patients with first-line medication.2018Guidelines for the diagnosis and treatment of lung cancer issued in Japan in recommend osimertinib high-levelEGFRPositive non-small cell lung cancer patients with first-line medication.2019Beginning of YearESMOfficial Journal of Oncology Yearbook (Annals of Oncology) "published" Pan-Asian TransferNSCLCClinical Practice Guidelines give priority to recommend osimertinib as the first-line treatment of choice.
The most critical efficacy evaluation index of anticancer drugs is to prolong the overall survival of patients (OS), improve the quality of life,OSIt is now widely accepted as the gold standard for evaluating the efficacy of anticancer drugs.2019Year8Month9AstraZeneca announcedFLAURAThe study reachedOSThe secondary endpoint of first-line treatment with osimertinibEGFRlate mutation positiveNSCLCpatients compared to the standardTKIstreatment showed statistical and clinical significanceOSimprovement, making osimertinib a U.FDAFirst Endorsed Extended Overall SurvivalEGFRTargeted drugs.FLAURAResearchOSThe results consolidate the position of osimertinib as first-line therapy.
From2015Year11Monthly AmericanFDASince its approval, Osimertinib has been widely used in the United States, Europe, Japan, China, Canada, Sweden, Israel, Mexico, Australia, etc.47countries and regions listed. Market sales have grown steadily,2017Annual Sales9.55billion dollars,2018years ago9Monthly Sales12.66Billion US dollars (+94%),2019YearH1Performance Sales14.14billion dollars (growth86%)。

At present, China has been approved for listing three generations.EGFR-TKIsOsimertinib is the only one, which is also currentlyFDA,EMAThe only approved three generationsEGFR-TKIs. Osimertinib was officially approved in ChinaNSCLCFirst-line therapy means:30The only thing that can really prolong survival in yearsEGFRTargeted drugs will further benefit domestic patients, can be described as ChinaEGFRA major milestone in the treatment of lung cancer!
Drug Introduction
Common name: osimertinib mesylate tablets
Commodity Name:Tagrix®(Terrisa®)
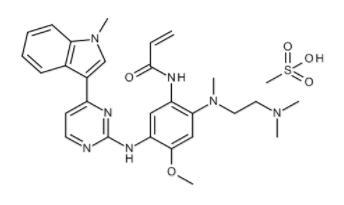
Molecular formula:C28H33N7O2·CH4O3S
Chemical formula:
Domestic, egfr, lung cancer, patient, first-line, treatment, mutation, cell, positive, nsclc

