Wuhan virus registered patents, the epidemic is currently causing great controversy!
Release time:
2020-02-14 15:14
The Wuhan Institute of Virology, Chinese Academy of Sciences (hereinafter referred to as "Wuhan Institute of Virology") announced on its website on February 4 that it had carried out joint research with the Academy of Military Medicine of the Academy of Military Sciences and made important progress in the screening of drugs to inhibit the 2019 of the new coronavirus. Its research shows that Redivevir and chloroquine phosphate can effectively inhibit the infection of the new coronavirus at the cellular level. The Wuhan Institute of Virology declared a Chinese invention patent (the use of anti -2019 novel coronavirus) on January 21, and will enter major countries in the world through the PCT route.
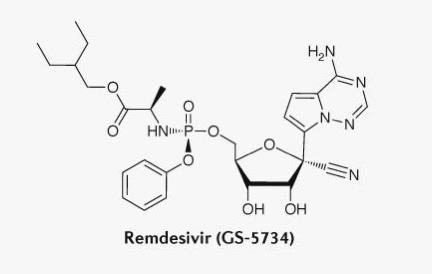
Redsivir is a nucleotide analog compound developed by Gilead Sciences (hereinafter referred to as "Gilead"), which has been patented in China and other parts of the world. The compound can inhibit RNA-dependent RNA synthetase (RdRp), which was originally developed for Ebola virus.
The act of applying for a patent by Wuhan virus has aroused extensive discussion, and there are many criticisms both inside and outside the industry. Guangming Daily said in its comments that only by meeting the hopes of the people can it be possible to make money in the development of drugs, and this order cannot be reversed. There are also people in the industry who support the view that if Wuhan virus Institute holds a patent for use, it is equivalent to having a bargaining chip, which can lower the purchase price of Redseway through cross-licensing, so it is necessary to apply for a patent.
On February 8, Gilead officials responded to the topic of clinical trials, supply and patents of radecivir. Patent applications have been filed in China and other countries claiming to target the use of raltegravir in coronavirus, and patent applications in China are still under review. Gilead believes that it is too early to discuss any compulsory or other types of licensing at this stage, and Gilead has not had any discussions with regulators about the cost of production and supply of patents or financial returns.
The relevant patents of Gilead in China shall refer to the Chinese patent application with application number 201680066796.8, which specifies the coronavirus in the claims, including SARS, MERS, 229E, NL63, OC43 and HKU1. The new coronavirus is its lower concept. At present, it is not known what the patent application technical scheme of Wuhan Virus Institute is, and it is not yet possible to evaluate whether the relevant patent application of Wuhan Virus Institute can be authorized.
At the same time, Wuhan Virus Institute also stated that if relevant foreign enterprises intend to contribute to the prevention and control of the epidemic in China, we both agreed not to require the implementation of the rights claimed by the patent if the country needs it, and hope to work with foreign pharmaceutical companies to make a modest contribution to the prevention and control of the epidemic. Gilead also responded that Redsivir is a investigational drug whose safety and effectiveness as a treatment for the new coronavirus have not yet been determined, and is doing everything possible to communicate and cooperate closely with officials in China, the United States and the World Health Organization.
Current, virus, wuhan, geely, china, patent, patent application, coronavirus, research, research and development

